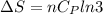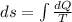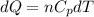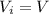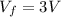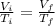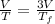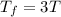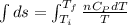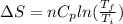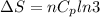Chemistry, 03.12.2019 21:31 squidmeat12
Asample consisting of n mol of an ideal gas undergoes a reversible isobaric expansion from volume vi to volume 3vi. find the change in entropy of the gas by calculating, ∫dq / t, where dq = ncpdt. (use the following as necessary: cp and n.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Asample consisting of n mol of an ideal gas undergoes a reversible isobaric expansion from volume vi...
Questions

Mathematics, 01.06.2021 21:30

Chemistry, 01.06.2021 21:30


Mathematics, 01.06.2021 21:30


Computers and Technology, 01.06.2021 21:30

Mathematics, 01.06.2021 21:30


Mathematics, 01.06.2021 21:30

Chemistry, 01.06.2021 21:30

Mathematics, 01.06.2021 21:30


Mathematics, 01.06.2021 21:30


Mathematics, 01.06.2021 21:30



Mathematics, 01.06.2021 21:30


Mathematics, 01.06.2021 21:30