
Chemistry, 03.12.2019 21:31 courtnicollee
Fe(ii) can be precipitated from a slightly basic (aq) solution by bubbling oxygen through the solution, which converts fe(ii) to insoluble fe(iii): 4fe(oh)+(aq)+ 4oh-(aq)+o2(g)+2h2o(l)=4fe(oh)3(s)h ow many grams of o2 are consumed to precipitate all of the iron in 85ml of 0.075 m fe(ii).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Fe(ii) can be precipitated from a slightly basic (aq) solution by bubbling oxygen through the soluti...
Questions




History, 08.12.2019 07:31

Mathematics, 08.12.2019 07:31






Mathematics, 08.12.2019 07:31

Biology, 08.12.2019 07:31



Mathematics, 08.12.2019 07:31

Mathematics, 08.12.2019 07:31


Mathematics, 08.12.2019 07:31


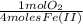 = 1,6x10⁻³ moles of O₂(g)
= 1,6x10⁻³ moles of O₂(g)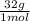 = 0,051g of O₂ are consumed
= 0,051g of O₂ are consumed

