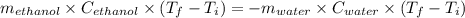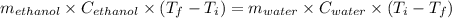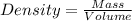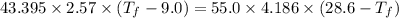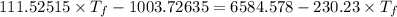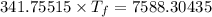Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1


Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
If 55.0 ml of ethanol (density=0.789g/ml)) initially at 9.0 ∘c is mixed with 55.0 ml of water (densi...
Questions



Mathematics, 09.02.2021 01:00

English, 09.02.2021 01:00






Mathematics, 09.02.2021 01:00

Mathematics, 09.02.2021 01:00




Mathematics, 09.02.2021 01:00

Chemistry, 09.02.2021 01:00