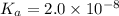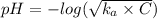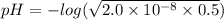Chemistry, 03.12.2019 17:31 Terrilady5
A0.50 m solution of an unknown acid has a ph = 4.0. of the following, which is the acid in the solution?
hocl (ka = 2.0 x 10-8)
hbr (strong acid)
hf (ka = 6.8 x 10-4)
c6h5oh (ka = 1.0 x 10-10)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1
You know the right answer?
A0.50 m solution of an unknown acid has a ph = 4.0. of the following, which is the acid in the solut...
Questions











Mathematics, 10.03.2020 08:59







English, 10.03.2020 09:00


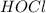 ,
,