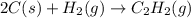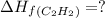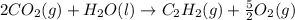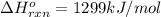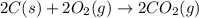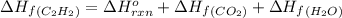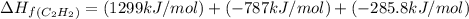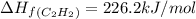The enthalpy of combustion of acetylene c2h2 is described by
c2h2 (g) + (5/2)o2 (g) > > > > > > > co2 (g) + h2o (l) heat of reaction (rxn) = -1299kj/mol
calculate the enthalpy of formation of accetylene, given the following enthalpies of formation
standard formation [co2 (g)]= -393.5 kj/mol
standard formation [h2o (l)] = -285.8 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

You know the right answer?
The enthalpy of combustion of acetylene c2h2 is described by
c2h2 (g) + (5/2)o2 (g) >...
c2h2 (g) + (5/2)o2 (g) >...
Questions







Mathematics, 02.07.2020 04:01

Mathematics, 02.07.2020 04:01

Mathematics, 02.07.2020 04:01



Mathematics, 02.07.2020 04:01

Mathematics, 02.07.2020 04:01


Mathematics, 02.07.2020 04:01

Mathematics, 02.07.2020 04:01


Mathematics, 02.07.2020 04:01


Mathematics, 02.07.2020 04:01

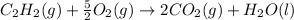
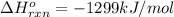
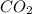 will be,
will be,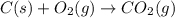
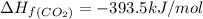
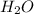 will be,
will be,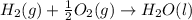
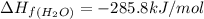
 will be,
will be,