
Two flasks of equal volume and at the same temperature contain different gases. one flask contains 5.0 g of o2, and the other flask contains 5.0 g of h2. is each of the following statements true or false? explain.
a) true. because the gases have the same volumes, they must have the same number of molecules.
b) false. because the molar mass of o2 is greater than the molar mass of h2, 5.0g of o2 will contain fewer molecules than 5.0 g of h2.
c)false. depending on the pressure each flask may contain different numbers of molecules.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
You know the right answer?
Two flasks of equal volume and at the same temperature contain different gases. one flask contains 5...
Questions

Mathematics, 09.03.2021 21:20


Mathematics, 09.03.2021 21:20

Mathematics, 09.03.2021 21:20



Mathematics, 09.03.2021 21:20

Arts, 09.03.2021 21:20

English, 09.03.2021 21:20

Spanish, 09.03.2021 21:20


Mathematics, 09.03.2021 21:20


English, 09.03.2021 21:20




Mathematics, 09.03.2021 21:20

Mathematics, 09.03.2021 21:20

Mathematics, 09.03.2021 21:20

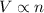
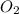 =
= 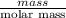
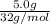
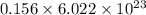
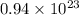 molecules
molecules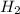 =
= 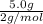
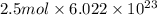
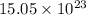 molecules
molecules![O_{2} will contain same molecules as 5.0 g of [tex]H_{2}](/tpl/images/0400/5815/5c250.png) is not true.
is not true.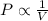 (at constant temperature and number of moles)
(at constant temperature and number of moles)

