
Chemistry, 03.12.2019 05:31 summerjoiner
Asample of 0.495 grams of solid khp is weighed into an erlenmeyer flask. this sample is titrated with a sodium hydroxide solution, and 28.56 ml of naoh are required to reach the endpoint. the sodium hydroxide solution is then used to titrate a sample of phosphoric acid of unknown concentration. it requires 29.88 ml of naoh to react with 10.33 ml of h3po4 solution. what is the concentration of the phosphoric acid?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 08:10
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
You know the right answer?
Asample of 0.495 grams of solid khp is weighed into an erlenmeyer flask. this sample is titrated wit...
Questions


History, 09.09.2021 22:00



Mathematics, 09.09.2021 22:00




Mathematics, 09.09.2021 22:00



Arts, 09.09.2021 22:00


History, 09.09.2021 22:00


Mathematics, 09.09.2021 22:00



Biology, 09.09.2021 22:00

Arts, 09.09.2021 22:00

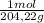 = 2,42x10⁻³ moles of KHP
= 2,42x10⁻³ moles of KHP

