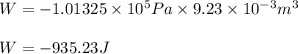Chemistry, 03.12.2019 04:31 jasmine2919
Automobile airbags contain solid sodium azide, nan 3 , that reacts to produce nitrogen gas when heated, thus inflating the bag. 2 nan 3 ( s ) ⟶ 2 na ( s ) + 3 n 2 ( g ) calculate the value of work, w , for the system if 16.5 g nan 3 reacts completely at 1.00 atm and 22 ∘ c.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
Automobile airbags contain solid sodium azide, nan 3 , that reacts to produce nitrogen gas when heat...
Questions




English, 18.03.2021 01:10



Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10


English, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10

English, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10

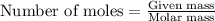
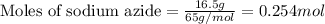
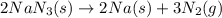
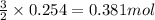 of nitrogen gas
of nitrogen gas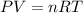
![22^oC=[22+273]K=295K](/tpl/images/0400/4731/7919f.png)
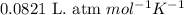
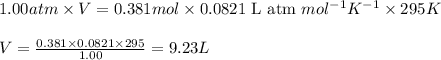
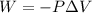
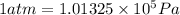 (Conversion factor: 1 atm = 101325 Pa)
(Conversion factor: 1 atm = 101325 Pa)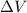 = change in volume =
= change in volume = 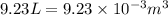 (Conversion factor:
(Conversion factor: 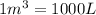 )
)