

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
What is the value for the reaction: n2(g) + 2 o2(g) --> n2o4(g) in terms of k values from the r...
Questions


History, 22.09.2019 12:50






Mathematics, 22.09.2019 12:50

Biology, 22.09.2019 12:50


Mathematics, 22.09.2019 12:50

History, 22.09.2019 12:50


History, 22.09.2019 12:50

Mathematics, 22.09.2019 12:50



Mathematics, 22.09.2019 12:50


Biology, 22.09.2019 12:50

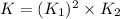
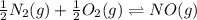
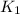
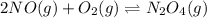
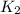
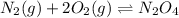
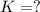
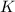 for the final reaction.
for the final reaction.

