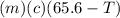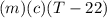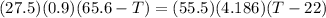Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
A27.5 −g aluminum block is warmed to 65.6 ∘c and plunged into an insulated beaker containing 55.5 g...
Questions

Biology, 28.09.2019 09:50

Mathematics, 28.09.2019 09:50





Mathematics, 28.09.2019 09:50

Biology, 28.09.2019 09:50


Biology, 28.09.2019 09:50




Chemistry, 28.09.2019 09:50

Social Studies, 28.09.2019 09:50