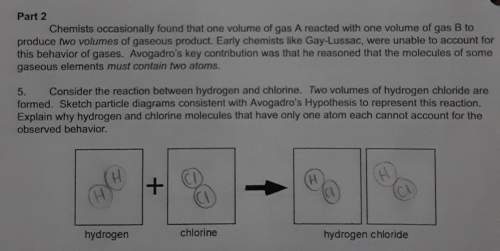
Consider the reaction between hydrogen and chlorine. two volumes of hydrogen chloride are
forme...

Consider the reaction between hydrogen and chlorine. two volumes of hydrogen chloride are
formed. sketch particle diagrams consistent with avogadro's hypothesis to represent this reaction
explain why hydrogen and chlorine molecules that have only one atom each cannot account for the
observed behavior.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
Questions

Mathematics, 10.02.2021 01:00

History, 10.02.2021 01:00





Arts, 10.02.2021 01:00

Health, 10.02.2021 01:00





Social Studies, 10.02.2021 01:00




Mathematics, 10.02.2021 01:00


Mathematics, 10.02.2021 01:00

Mathematics, 10.02.2021 01:00



