

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
Given thath2(g) + f2(g) -> 2hf(g) => ∆h = -546.6 kj . mol-12h2(g) + o2(g) -> 2h20(l) =&g...
Questions

Mathematics, 03.08.2020 14:01

Biology, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01








History, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01



Chemistry, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01


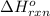 for the reaction is -521.6 kJ.
for the reaction is -521.6 kJ.
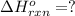
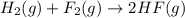
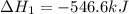 ( × 2)
( × 2)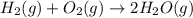
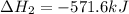
![\Delta H^o_{rxn}=[2\times \Delta H_1]+[1\times (-\Delta H_2)]](/tpl/images/0400/0579/648b7.png)
![\Delta H^o_{rxn}=[(2\times (-546.6))+(1\times (571.6))]=-521.6kJ](/tpl/images/0400/0579/83f77.png)


