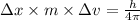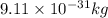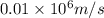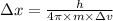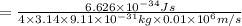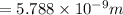Chemistry, 03.12.2019 00:31 Sariyahhall1
German physicist werner heisenberg related the uncertainty of an object's position ( δ x ) (δx) to the uncertainty in its velocity ( δ v ) (δv) δ x ≥ h 4 π m δ v δx≥h4πmδv where h h is planck's constant and m m is the mass of the object. the mass of an electron is 9.11 × 10 − 31 kg. 9.11×10−31 kg. what is the uncertainty in the position of an electron moving at 6.00 × 10 6 m/s 6.00×106 m/s with an uncertainty of δ v = 0.01 × 10 6 m/s ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 1
You know the right answer?
German physicist werner heisenberg related the uncertainty of an object's position ( δ x ) (δx) to t...
Questions

Chemistry, 17.03.2020 22:09




English, 17.03.2020 22:09







Mathematics, 17.03.2020 22:09


Social Studies, 17.03.2020 22:09

Mathematics, 17.03.2020 22:09



Mathematics, 17.03.2020 22:09

History, 17.03.2020 22:09

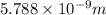 is the uncertainty in the position of a moving electron.
is the uncertainty in the position of a moving electron.