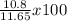Chemistry, 02.12.2019 22:31 miguelturner
An experiment reacts 20.4 g of zinc metal with a solution containing an excess of iron (iii) sulfate. after the reaction, 10.8 grams of iron metal are recovered. what is the percent yield of the experiment?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1
You know the right answer?
An experiment reacts 20.4 g of zinc metal with a solution containing an excess of iron (iii) sulfate...
Questions




History, 22.06.2019 23:30



History, 22.06.2019 23:30

Health, 22.06.2019 23:30



English, 22.06.2019 23:30

Biology, 22.06.2019 23:30



Social Studies, 22.06.2019 23:30




Computers and Technology, 22.06.2019 23:30