
Chemistry, 02.12.2019 21:31 krystalhurst97
Using the standard enthalpies of formation for the chemicals involved, calculate the enthalpy change for the following reaction.
(note: show the math clearly and provide units in your set up) ( hf values in kj/mol are as follows: no2 32, h2o 286, hno3 207, no 90.)
3no2(g) h2o(l) 2hno3(aq) no(g) g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 23.06.2019 18:50
Why are very high temperatures and pressures required for fusion to occur? to generate the neutrons that are needed to break the nuclei o to overcome the repulsion between the protons in the nuclei that join to maintain the proper conditions to keep the chain reaction going to keep the uranium fuel separate from the control rods
Answers: 1

Chemistry, 23.06.2019 19:30
Why does 4.03/0.0000035 = 1.2 x 106, instead of a different number of significant figures?
Answers: 1

You know the right answer?
Using the standard enthalpies of formation for the chemicals involved, calculate the enthalpy change...
Questions



English, 02.11.2020 23:30

Mathematics, 02.11.2020 23:30

History, 02.11.2020 23:30

Mathematics, 02.11.2020 23:30


Mathematics, 02.11.2020 23:30






Mathematics, 02.11.2020 23:30

Mathematics, 02.11.2020 23:30




Chemistry, 02.11.2020 23:30

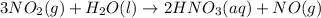
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0399/7869/76c37.png)
![\Delta H=[(n_{HNO_3}\times \Delta H_{HNO_3})+(n_{NO}\times \Delta H_{NO})]-[(n_{H_2O}\times \Delta H_{H_2O})+(n_{NO_2}\times \Delta H_{NO_2})]](/tpl/images/0399/7869/7081c.png)
![\Delta H=[(2\times -207)+(1\times 90)]-[(1\times -286)+(3\times 32)]](/tpl/images/0399/7869/1d6ad.png)



