Chemistry, 02.12.2019 18:31 hayesvolcano
Write the half-reactions as they occur at each electrode and the net cell reaction for this electrochemical cell containing copper and silver. cu ( s ) ∣ ∣ cu 2 + ( aq ) ∥ ∥ ag + ( aq ) ∣ ∣ ag ( s ) anode: cathode: net cell reaction:

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 11:30
If this sedimentary rock layer is truly the oldest one of marine origin, what do you think that tells usabout the formation of earth's oceans?
Answers: 2

Chemistry, 23.06.2019 11:50
What is the oxidation half-reaction for this unbalanced redox equation? cr2o72– + fe2+ → cr3+ + fe3+ cr3+ → cr2o72– cr2o72– → cr3+ fe3+ → fe2+ fe2+ → fe3+?
Answers: 2

Chemistry, 23.06.2019 14:00
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature.
Answers: 1
You know the right answer?
Write the half-reactions as they occur at each electrode and the net cell reaction for this electroc...
Questions

Health, 21.01.2021 20:50

Mathematics, 21.01.2021 20:50


Mathematics, 21.01.2021 20:50


Physics, 21.01.2021 20:50

Mathematics, 21.01.2021 20:50


Mathematics, 21.01.2021 20:50

Chemistry, 21.01.2021 20:50






Mathematics, 21.01.2021 20:50



Chemistry, 21.01.2021 20:50

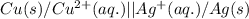
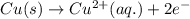
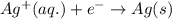 ( × 2)
( × 2)