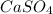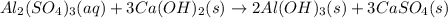Given the unbalanced equation:
- al(so )3 + - ca(oh)2 +_ al(oh)3 + caso,
what is the coef...

Chemistry, 01.12.2019 09:31 trinityparrish47
Given the unbalanced equation:
- al(so )3 + - ca(oh)2 +_ al(oh)3 + caso,
what is the coefficient in front of the caso, when the equation is completely balanced with
the smallest whole-number coefficients?
a. 1
b.2
c.3
d.4

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

You know the right answer?
Questions


Health, 28.10.2020 21:40

Mathematics, 28.10.2020 21:40




Mathematics, 28.10.2020 21:40

History, 28.10.2020 21:40

Mathematics, 28.10.2020 21:40



History, 28.10.2020 21:40




Biology, 28.10.2020 21:40

History, 28.10.2020 21:40

Mathematics, 28.10.2020 21:40