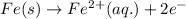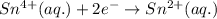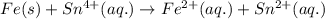Chemistry, 30.11.2019 07:31 vanessa051266
In the electrochemical cell using the redox reaction below, the anode half reaction is sn4+ (aq) + fe (s) → sn2+ (aq) + fe2+ (aq) in the electrochemical cell using the redox reaction below, the anode half reaction is (aq) + (s) (aq) + (aq) fe→fe2++2e− sn4+→sn2++2e− fe+2e−→fe2+ sn4++2e−→sn2+ fe+2e−→sn2+ request answer

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
In the electrochemical cell using the redox reaction below, the anode half reaction is sn4+ (aq) +...
Questions





Geography, 12.07.2019 09:00

Social Studies, 12.07.2019 09:00

Chemistry, 12.07.2019 09:00

History, 12.07.2019 09:00

Social Studies, 12.07.2019 09:00


Mathematics, 12.07.2019 09:00


Biology, 12.07.2019 09:00

English, 12.07.2019 09:00

Mathematics, 12.07.2019 09:00





Mathematics, 12.07.2019 09:00