
Chemistry, 30.11.2019 06:31 Virnalis1112
What is the [h3o+] and the ph of a benzoic acid-benzoate buffer that consists of 0.17 m c6h5cooh and 0.42 m c6h5coona? (ka of benzoic acid = 6.3 × 10^−5). be sure to report your answer to the correct number of significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
What is the [h3o+] and the ph of a benzoic acid-benzoate buffer that consists of 0.17 m c6h5cooh and...
Questions

Mathematics, 06.07.2019 05:00

Advanced Placement (AP), 06.07.2019 05:00

Biology, 06.07.2019 05:00

History, 06.07.2019 05:00

English, 06.07.2019 05:00

Mathematics, 06.07.2019 05:00

Mathematics, 06.07.2019 05:00



English, 06.07.2019 05:00

Biology, 06.07.2019 05:00

Biology, 06.07.2019 05:00

Mathematics, 06.07.2019 05:00

History, 06.07.2019 05:00



Mathematics, 06.07.2019 05:00



Mathematics, 06.07.2019 05:00

![[H_{3}O^{+}]=x M = 2.5\times 10^{-5}M](/tpl/images/0397/1268/ab31e.png) and pH = 4.6
and pH = 4.6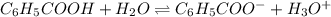
![\frac{[C_{6}H_{5}COO^{-}][H_{3}O^{+}]}{[C_{6}H_{5}COOH]}=K_{a}(C_{6}H_{5}COOH)](/tpl/images/0397/1268/75106.png)
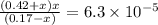
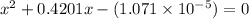
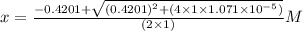
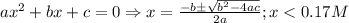 )
)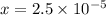 M
M![pH=-log[H_{3}O^{+}]=-logx=-log(2.5\times 10^{-5})=4.6](/tpl/images/0397/1268/f0ff1.png)


