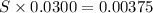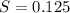What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is required to completely react with 25.00 ml of a 0.300 m thiosulfate solution, k2s2o3(aq)? the chemical equation for the reaction is 2 s2o32-(aq) + i3-(aq) → s4o62-(aq) + 3 i-(aq).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 23.06.2019 09:00
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2

Chemistry, 23.06.2019 16:40
Which statement is true about market economies? government goals drive business decisions. people have the freedom to choose their jobs. several are market economies vist around the world
Answers: 2
You know the right answer?
What is the molarity of a potassium triiodide solution, ki3(aq), if 30.00 ml of the solution is requ...
Questions





Health, 20.10.2020 21:01

Social Studies, 20.10.2020 21:01








Physics, 20.10.2020 21:01


History, 20.10.2020 21:01

Chemistry, 20.10.2020 21:01



Physics, 20.10.2020 21:01

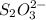 completely react with 1 mol of
completely react with 1 mol of 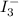 .
.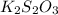 solution =
solution = 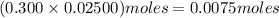
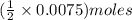 of
of 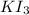 solution is S (M) then-
solution is S (M) then-