
Chemistry, 30.11.2019 05:31 ahicks2004
Consider the following generic chemical equation. 2a+4b→3c what is the limiting reactant when each of the following amounts of a and b are allowed to react? part a 2 mola; 5 molb 2 ; 5 b a submitrequest answer part b 1.8 mola; 4 molb 1.8 ; 4 b a submitrequest answer part c 3 mola; 4 molb 3 ; 4 b a submitrequest answer part d 22 mola; 40 molb 22 ; 40 b a

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Consider the following generic chemical equation. 2a+4b→3c what is the limiting reactant when each o...
Questions


History, 06.09.2020 03:01

English, 06.09.2020 03:01

Social Studies, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01



Chemistry, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01


Chemistry, 06.09.2020 03:01

Chemistry, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01

Chemistry, 06.09.2020 03:01



Mathematics, 06.09.2020 03:01


Mathematics, 06.09.2020 03:01

Computers and Technology, 06.09.2020 03:01

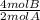 = 4moles of B
= 4moles of B

