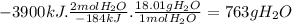Chemistry, 30.11.2019 05:31 HistoryLee
According to the following thermochemical equation, what mass of h2o (in g) must form in order to produce 3900 kj of energy?
sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh°rxn = -184 kj sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh°rxn = -184 kj 216 g 382 g 763 g 408 g 272 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

You know the right answer?
According to the following thermochemical equation, what mass of h2o (in g) must form in order to pr...
Questions


History, 23.09.2019 02:00

English, 23.09.2019 02:10

Mathematics, 23.09.2019 02:10


Computers and Technology, 23.09.2019 02:10

Physics, 23.09.2019 02:10

Physics, 23.09.2019 02:10

Biology, 23.09.2019 02:10

History, 23.09.2019 02:10



Mathematics, 23.09.2019 02:10


Mathematics, 23.09.2019 02:10

Physics, 23.09.2019 02:10


Mathematics, 23.09.2019 02:10


Chemistry, 23.09.2019 02:10