
Chemistry, 30.11.2019 03:31 emily200705
If the equilibrium constant for a reaction is 0.00010, what does this tell us about the position of equilibrium for that reaction?
a. there are more reactants than products at equilibrium.
b. there are more products than reactants at equilibrium.
c. at equilibrium, the concentration of reactants is the same as that of the products.
d. the concentration of the product at equilibrium is 0.00010 m.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
If the equilibrium constant for a reaction is 0.00010, what does this tell us about the position of...
Questions

Mathematics, 18.02.2021 20:00



Mathematics, 18.02.2021 20:00

Mathematics, 18.02.2021 20:00

Social Studies, 18.02.2021 20:00

Mathematics, 18.02.2021 20:00


Mathematics, 18.02.2021 20:00

Business, 18.02.2021 20:00




Mathematics, 18.02.2021 20:00



Arts, 18.02.2021 20:00

Computers and Technology, 18.02.2021 20:00

Mathematics, 18.02.2021 20:00

Biology, 18.02.2021 20:00

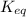
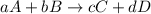
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0396/7601/9c8b0.png)
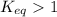 ; the reaction is product favored.
; the reaction is product favored.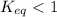 ; the reaction is reactant favored.
; the reaction is reactant favored.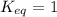 ; the reaction is in equilibrium.
; the reaction is in equilibrium.

