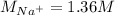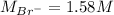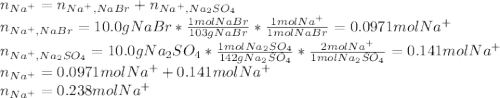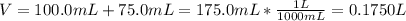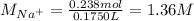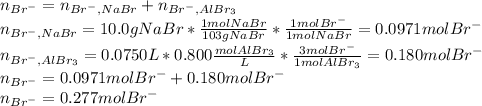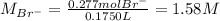Chemistry, 30.11.2019 03:31 caplode7497
Asolution is prepared by dissolving 10.0 g of nabr and 10.0 g of na2so4 in water to make a 100.0 ml solution. this solution is then mixed with 75.0 ml of a 0.800 m aqueous solution of albr3. calculate the concentration (m) of na+ and br− in the final solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 22.06.2019 05:30
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 10.0 g of nabr and 10.0 g of na2so4 in water to make a 100.0 ml...
Questions

Mathematics, 27.06.2019 04:00










History, 27.06.2019 04:00




History, 27.06.2019 04:00

Computers and Technology, 27.06.2019 04:00




History, 27.06.2019 04:00