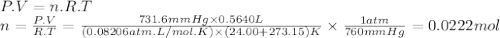Chemistry, 30.11.2019 03:31 fattypickeltoefungus
Oxygen gas can be prepared by heating potassium chlorate: 2kclo3(s)2kcl(s) + 3o2(g) in one experiment, a sample of kclo3 reacts and the gas produced is collected by water displacement. the gas sample has a temperature of 24.00 °c, a volume of 564.0 ml, and a pressure of 754.0 mm hg.
calculate the amount (in moles) of oxygen gas produced in the reaction. the vapor pressure of water is 22.38 mm hg at 24.00 °c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2
You know the right answer?
Oxygen gas can be prepared by heating potassium chlorate: 2kclo3(s)2kcl(s) + 3o2(g) in one experime...
Questions

Mathematics, 29.11.2021 08:00

Social Studies, 29.11.2021 08:00

Mathematics, 29.11.2021 08:00


History, 29.11.2021 08:00

Mathematics, 29.11.2021 08:00

Mathematics, 29.11.2021 08:00

History, 29.11.2021 08:00


Mathematics, 29.11.2021 08:00



Mathematics, 29.11.2021 08:00



English, 29.11.2021 08:00


Mathematics, 29.11.2021 08:00