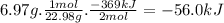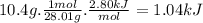The following thermochemical equation is for the reaction of sodium(s) with water(l) to form sodium hydroxide(aq) and hydrogen(g). 2na(s) + 2h2o(l)2naoh(aq) + h2(g) h = -369 kj when 6.97 grams of sodium(s) react with excess water(l), kj of energy are .
2) the following thermochemical equation is for the reaction of carbon monoxide(g) with water(l) to form carbon dioxide(g) and hydrogen(g).
co(g) + h2o(l)arrow. gif co2(g) + h2(g) delta16-1.gifh = 2.80 kj
when 10.4 grams of carbon monoxide(g) react with excess water(l), kj of energy are .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

You know the right answer?
The following thermochemical equation is for the reaction of sodium(s) with water(l) to form sodium...
Questions

History, 30.03.2021 05:40

Mathematics, 30.03.2021 05:40





Biology, 30.03.2021 05:40

Health, 30.03.2021 05:40

Computers and Technology, 30.03.2021 05:40



Mathematics, 30.03.2021 05:40



Computers and Technology, 30.03.2021 05:40



Mathematics, 30.03.2021 05:40


Mathematics, 30.03.2021 05:40