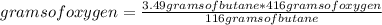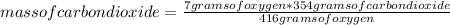Chemistry, 30.11.2019 03:31 fespinoza019
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. suppose 3.49 g of butane is mixed with 7.0 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. s...
Questions


Mathematics, 16.01.2020 04:31

Mathematics, 16.01.2020 04:31



Biology, 16.01.2020 04:31

Mathematics, 16.01.2020 04:31

History, 16.01.2020 04:31

Mathematics, 16.01.2020 04:31

Geography, 16.01.2020 04:31


World Languages, 16.01.2020 04:31

Mathematics, 16.01.2020 04:31



Mathematics, 16.01.2020 04:31

Mathematics, 16.01.2020 04:31

Mathematics, 16.01.2020 04:31

Mathematics, 16.01.2020 04:31

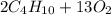 ⇒
⇒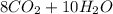
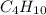 : 12 g/mol *4 + 1 g/mol *10= 58 g/molO₂: 16 g/mol *2= 32 g/molCO₂: 12 g/mol + 16 g/mol *2= 44 g/molH₂O: 1 g/mol *2 + 16 g/mol= 18 g/mol
: 12 g/mol *4 + 1 g/mol *10= 58 g/molO₂: 16 g/mol *2= 32 g/molCO₂: 12 g/mol + 16 g/mol *2= 44 g/molH₂O: 1 g/mol *2 + 16 g/mol= 18 g/mol