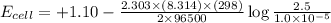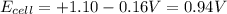The standard cell potential (e°cell) for the reaction below is +1.10v. the cell potential for this reaction is v when the concentration of [cu2+]=1.0⋅10−5m and [zn2+]=2.5m. zn (s) + cu2+ (aq) → cu (s) + zn2+ (aq) the standard cell potential () for the reaction below is . the cell potential for this reaction is when the concentration of and (s) + (aq) (s) + (aq) 0.78 1.10 0.94 1.26 1.42

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2
You know the right answer?
The standard cell potential (e°cell) for the reaction below is +1.10v. the cell potential for this r...
Questions

History, 05.05.2020 01:30

Mathematics, 05.05.2020 01:30

Mathematics, 05.05.2020 01:30


Biology, 05.05.2020 01:30

English, 05.05.2020 01:30




Mathematics, 05.05.2020 01:30



Mathematics, 05.05.2020 01:30

Chemistry, 05.05.2020 01:30

Mathematics, 05.05.2020 01:30


Mathematics, 05.05.2020 01:30


Social Studies, 05.05.2020 01:30

Mathematics, 05.05.2020 01:30

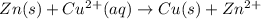
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]}{[Cu^{2+}]}](/tpl/images/0396/8274/2e6f5.png)
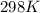
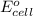 = standard electrode potential of the cell = +1.10 V
= standard electrode potential of the cell = +1.10 V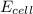 = emf of the cell = ?
= emf of the cell = ?