
Chemistry, 30.11.2019 02:31 aylinkayla
Avoltaic cell is constructed with two silver-silver chloride electrodes, each of which is based on the following half-reaction:
agcl(s)+e−→ag(s)+cl−(aq).
the two cell compartments have [cl−]=1.42×10−2 m and [cl−]= 2.00 m , respectively.
part a
which electrode is the cathode of the cell?
the compartment with 1.42×10−2 m cl(aq) is the cathode
the compartment with 2.00 m cl(aq) is the cathode
part b
what is the standard emf of the cell?
part c
what is the cell emf for the concentrations given?
part d
for the anode compartment, predict whether will increase, decrease, or stay the same as the cell operates.
increase
decrease
stay the same
part e
for the cathode compartment, predict whether will increase, decrease, or stay the same as the cell operates.
increase
decrease
stay the same

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
Avoltaic cell is constructed with two silver-silver chloride electrodes, each of which is based on t...
Questions


History, 20.01.2021 01:10


Mathematics, 20.01.2021 01:10


Social Studies, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10


Mathematics, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10



Mathematics, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10


 total) formed by the system , AgCl(s)+e−→Ag(s)+Cl−(aq) is defined as the standard electrode potential of the right-hand electrode, minus the standard electrode potential of the left-hand electrode.
total) formed by the system , AgCl(s)+e−→Ag(s)+Cl−(aq) is defined as the standard electrode potential of the right-hand electrode, minus the standard electrode potential of the left-hand electrode.
 )
) ) silver gas and
) silver gas and 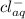 aqueous chloride ion.
aqueous chloride ion.


