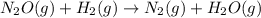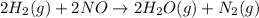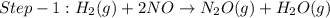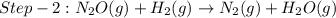Chemistry, 30.11.2019 01:31 uticabadgirl
The reduction of nitrogen monoxide is described by the following chemical equation: 2h2 (g) +2no (g) 2h20 ()n2 (g suppose a two-step mechanism is proposed for this reaction, beginning with this elementary reaction: h2 g+2no(g)- n20 (g)+h20(g) suppose also that the second step of the mechanism should be bimolecular suggest a reasonable second step. that is, write the balanced chemical equation of a bimolecular elementary reaction that would complete the proposed mechanism

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2


Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
The reduction of nitrogen monoxide is described by the following chemical equation: 2h2 (g) +2no (g...
Questions



Mathematics, 27.09.2019 16:10



Mathematics, 27.09.2019 16:10







History, 27.09.2019 16:10




History, 27.09.2019 16:10