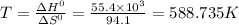Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2
You know the right answer?
For a particular reaction, δ h ∘ = 55.4 kj δh∘=55.4 kj and δ s ∘ = 94.1 j/k. δs∘=94.1 j/k. assuming...
Questions


Mathematics, 01.03.2021 21:30

Arts, 01.03.2021 21:30


Physics, 01.03.2021 21:30



Chemistry, 01.03.2021 21:30




Mathematics, 01.03.2021 21:30

Chemistry, 01.03.2021 21:30

Mathematics, 01.03.2021 21:30

History, 01.03.2021 21:30


Mathematics, 01.03.2021 21:30



Mathematics, 01.03.2021 21:30

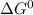 .
.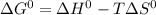
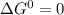 as the limiting condition.
as the limiting condition.