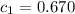Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
When a 40.0 g sample of a metal at 25.00 °c is added to 65.0 g of water at 100.00 °c, the final temp...
Questions

Mathematics, 09.06.2021 15:50



Geography, 09.06.2021 15:50

Mathematics, 09.06.2021 15:50

Mathematics, 09.06.2021 15:50


Mathematics, 09.06.2021 15:50







History, 09.06.2021 15:50


Mathematics, 09.06.2021 15:50

Mathematics, 09.06.2021 15:50

Biology, 09.06.2021 15:50

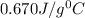
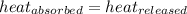
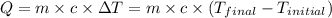
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0396/4522/09236.png) .................(1)
.................(1)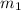 = mass of metal = 40.0 g
= mass of metal = 40.0 g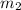 = mass of water = 65.0 g
= mass of water = 65.0 g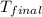 = final temperature =
= final temperature = 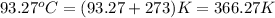
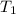 = temperature of metal =
= temperature of metal = 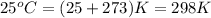
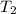 = temperature of water =
= temperature of water = 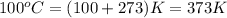
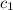 = specific heat of metal = ?
= specific heat of metal = ?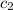 = specific heat of water=
= specific heat of water= 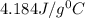
![40.0\times c_1\times (366.27-298)=-[65.0\times 4.184\times (366.27-373)]](/tpl/images/0396/4522/88e12.png)