
Chemistry, 30.11.2019 00:31 jazmaine1217
The gas-phase reaction follows an elementary rate law and is to be carried out first in a pfr and then in a separate experiment in a cstr. when pure a is fed to a 10 dm 3 pfr at 300 k and a volumetric flow rate of 5 dm 3 /s, the conversion is 80%. when a mixture of 50% a and 50% inert (i) is fed to a 10 dm 3 cstr at 320 k and a volumetric flow rate of 5 dm 3 /s, the conversion is also 80%. what is the activation energy in cal/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
The gas-phase reaction follows an elementary rate law and is to be carried out first in a pfr and th...
Questions

Mathematics, 29.01.2020 06:43


Mathematics, 29.01.2020 06:43

Engineering, 29.01.2020 06:43


Mathematics, 29.01.2020 06:43

Mathematics, 29.01.2020 06:43


Mathematics, 29.01.2020 06:43










English, 29.01.2020 06:43

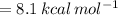
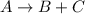
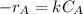
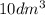

![V= \frac{v_{0}}{k}[(1+\epsilon )ln(\frac{1}{1-X}-\epsilon X)]](/tpl/images/0396/4811/340fc.png)
![k= \frac{v_{0}}{V}[(1+\epsilon )ln(\frac{1}{1-X}-\epsilon X)]............(1)](/tpl/images/0396/4811/d091c.png)
 is 1.
is 1.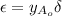
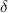 = change in total number of moles per mole of A reacte.
= change in total number of moles per mole of A reacte.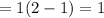
![k=\frac{5m^{3}/s}{10dm^{3}}[(1+1)ln \frac{1}{1-0.8}-1 \times 0.8] = 1.2s^{-1}](/tpl/images/0396/4811/db55d.png)
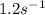
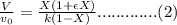
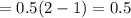
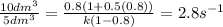
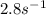
![k(T_{2})=k(T_{1})exp[\frac{E}{R}(\frac{1}{T_{1}}-\frac{1}{T_{2}})]](/tpl/images/0396/4811/6be68.png)




