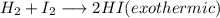Chemistry, 29.11.2019 07:31 heathkid23
 + i_2(g) \longrightarrow 2hi(g))
the forward reaction above is exothermic. at equilibrium, what happens if the reaction mixture is cooled at constant volume?
select all that apply.
1. the reaction absorbs energy.
2. the reaction releases energy.
3. [h₂] and [i₂] increase.
4. [h₂] and [i₂] decrease.
5. [h₂] and [i₂] remain constant.
6. [hi] increases.
7. [hi] decreases.
8. [hi] remains constant.
if c is added to the equilibrium system above, in which direction will the equilibrium shift?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2


You know the right answer?
[tex]h_2(g) + i_2(g) \longrightarrow 2hi(g)[/tex]
the forward reaction above is exothermic. at...
the forward reaction above is exothermic. at...
Questions

Arts, 01.09.2019 21:30

Business, 01.09.2019 21:30


History, 01.09.2019 21:30


History, 01.09.2019 21:30



Mathematics, 01.09.2019 21:30




Social Studies, 01.09.2019 21:30

Social Studies, 01.09.2019 21:30


English, 01.09.2019 21:30


Biology, 01.09.2019 21:30


Mathematics, 01.09.2019 21:30

![[H_{2}]](/tpl/images/0395/9023/f6f78.png) and
and ![[I_{2}]](/tpl/images/0395/9023/d0626.png) increase.
increase.![[HI]](/tpl/images/0395/9023/7d1e5.png) increases.
increases.