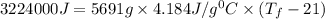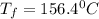The balanced combustion reaction for c 6 h 6 is 2 c 6 h 6 ( l ) 15 o 2 ( g ) ⟶ 12 co 2 ( g ) 6 h 2 o ( l ) 6542 kj if 7.700 g c 6 h 6 is burned and the heat produced from the burning is added to 5691 g of water at 21 ∘ c, what is the final temperature of the water

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
The balanced combustion reaction for c 6 h 6 is 2 c 6 h 6 ( l ) 15 o 2 ( g ) ⟶ 12 co 2 ( g ) 6 h 2 o...
Questions



Biology, 17.07.2020 21:01










Chemistry, 17.07.2020 21:01

Chemistry, 17.07.2020 21:01



Chemistry, 17.07.2020 21:01

Mathematics, 17.07.2020 21:01


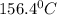
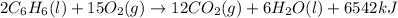
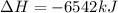
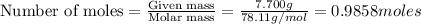
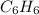 releases = 6542 kJ of heat
releases = 6542 kJ of heat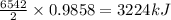 of heat
of heat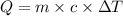
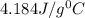
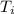 = 21.0°C
= 21.0°C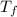 = ?
= ?