Chemistry, 29.11.2019 03:31 jordanmazer17
Enter your answer in the provided box. s(rhombic) + o2(g) → so2(g) δho rxn= −296.06 kj/mols(monoclinic) + o2(g) → so2(g) δho rxn= −296.36 kj/molcalculate the enthalpy change for the transformations(rhombic) → s(monoclinic)(monoclinic and rhombic are different allotropic forms of elemental /mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
You know the right answer?
Enter your answer in the provided box. s(rhombic) + o2(g) → so2(g) δho rxn= −296.06 kj/mols(monoclin...
Questions

Mathematics, 09.12.2021 21:40


Physics, 09.12.2021 21:40


Mathematics, 09.12.2021 21:40




Mathematics, 09.12.2021 21:40

Mathematics, 09.12.2021 21:40


Biology, 09.12.2021 21:40


Mathematics, 09.12.2021 21:40



Mathematics, 09.12.2021 21:40

Mathematics, 09.12.2021 21:40


Mathematics, 09.12.2021 21:40

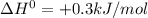 .
.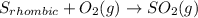
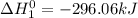 (1)
(1)
![S_{monoclinic}+O_2(g)\rightarrow SO_2(g)/tex] [tex]\Delta H^0_2=-296.36kJ](/tpl/images/0395/6241/b4124.png) (2)
(2)
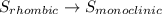
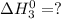 (3)
(3)