
Chemistry, 28.11.2019 22:31 leannadoughty06
A3.452 g sample containing an unknown amount of a ce(iv) salt is dissolved in 250.0-ml of 1 m h2so4. a 25.00 ml aliquot is analyzed by adding ki and titrating the i3– that forms with s2o32–. the end point was reached following the addition of 13.02 ml of 0.03428 m na2s2o3. calculate the weight percent of ce4 in the sample. when should the indicator be added to this titration? (at the beginning or just before the end point? )

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Type the correct answer in the box. spell all words correctly .what does biodiesel produce in higher amounts? biodiesel produces higher amounts
Answers: 2

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
A3.452 g sample containing an unknown amount of a ce(iv) salt is dissolved in 250.0-ml of 1 m h2so4....
Questions

History, 28.11.2020 14:00

Mathematics, 28.11.2020 14:00

Law, 28.11.2020 14:00


Mathematics, 28.11.2020 14:00

History, 28.11.2020 14:00


Biology, 28.11.2020 14:00

Geography, 28.11.2020 14:00

Mathematics, 28.11.2020 14:00

Chemistry, 28.11.2020 14:00

English, 28.11.2020 14:00

Mathematics, 28.11.2020 14:00

Business, 28.11.2020 14:00

Spanish, 28.11.2020 14:00


Mathematics, 28.11.2020 14:00


Biology, 28.11.2020 14:00

History, 28.11.2020 14:00

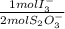 = 2,2315x10⁻⁴ moles of I₃⁻
= 2,2315x10⁻⁴ moles of I₃⁻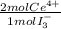 = 4,463x10⁻⁴ moles of Ce(IV). These moles are:
= 4,463x10⁻⁴ moles of Ce(IV). These moles are: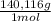 = 0,0625 g of Ce(IV)
= 0,0625 g of Ce(IV)

