
Chemistry, 28.11.2019 21:31 mervindavisk
Which of the following statements about the kinetic molecular theory are not correct?
a. the combined volume of all the molecules of the gas is negligible relative to the total volume in which the gas is contained
b. gases consist of molecules that are in continuous, random motion
c. the average kinetic energy of the molecules is not related to the absolute temperature
d. attractive and repulsive forces between gas molecules are negligible

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2
You know the right answer?
Which of the following statements about the kinetic molecular theory are not correct?
a. the...
a. the...
Questions


Mathematics, 13.01.2022 19:20




Mathematics, 13.01.2022 19:30

French, 13.01.2022 19:30

Chemistry, 13.01.2022 19:30

Mathematics, 13.01.2022 19:30

History, 13.01.2022 19:40






Mathematics, 13.01.2022 19:40




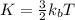
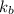 is the Boltzmann constant
is the Boltzmann constant

