

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 08:30
Plz a person walks 1 mile every day for exercise, leaving her front porch at 9 am and returning to her front porch at 9: 25 am what was the total displacement of her daily walk a. 1 mile b. 0 c. 25 min d. none of the above
Answers: 2

Chemistry, 23.06.2019 14:00
Cassandra made a venn diagram to compare and contrast the two stages of cellular respiration. which belongs in the area marked x? energy is released. oxygen is used up. glucose is broken down. carbon dioxide is used up.
Answers: 1
You know the right answer?
If you are given an ideal gas with pressure (p) = 259,392.00 pa and temperature (t) = 2.00 oc
...
...
Questions


World Languages, 16.09.2019 21:30



History, 16.09.2019 21:30

Mathematics, 16.09.2019 21:30

Chemistry, 16.09.2019 21:30

History, 16.09.2019 21:30

Arts, 16.09.2019 21:30

Mathematics, 16.09.2019 21:30

Social Studies, 16.09.2019 21:30

English, 16.09.2019 21:30





History, 16.09.2019 21:30



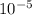 atm
atm

