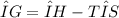Which of the following is true?
a. a reaction in which the entropy of the system increases ca...

Which of the following is true?
a. a reaction in which the entropy of the system increases can be spontaneous only if it is endothermic.
b. a reaction in which the entropy of the system decreases can be spontaneous only if it is endothermic.
c. a reaction in which the entropy of the system decreases can be spontaneous only if it is exothermic.
d. a reaction in which the entropy of the system increases can be spontaneous only if it is exothermic.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1


Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Questions


Biology, 06.07.2019 04:30

Biology, 06.07.2019 04:30





Biology, 06.07.2019 04:30





Social Studies, 06.07.2019 04:30

History, 06.07.2019 04:30

Geography, 06.07.2019 04:30


Geography, 06.07.2019 04:30

Mathematics, 06.07.2019 04:30

Mathematics, 06.07.2019 04:30

Mathematics, 06.07.2019 04:30