Chemistry, 28.11.2019 06:31 mxltie1651
A9.000l tank at 27.0°c is filled with 5.29g of sulfur tetrafluoride gas and 15.6g of carbon dioxide gas. you can assume both gases behave as ideal gases under these conditions. calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. round each of your answers to 3 significant digits. sulfur tetraflouride: mole fraction? partial pressure? carbon dioxide: mole fraction? partial pressure? total pressure in tank?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1


Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1
You know the right answer?
A9.000l tank at 27.0°c is filled with 5.29g of sulfur tetrafluoride gas and 15.6g of carbon dioxide...
Questions



English, 19.02.2020 04:02

Mathematics, 19.02.2020 04:02


Mathematics, 19.02.2020 04:02








Mathematics, 19.02.2020 04:02


Mathematics, 19.02.2020 04:02

Mathematics, 19.02.2020 04:02



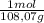 = 0,0489 moles SF₄
= 0,0489 moles SF₄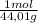 = 0,354 moles CO₂
= 0,354 moles CO₂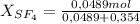 = 0,121
= 0,121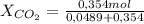 = 0,879
= 0,879