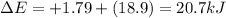Chemistry, 28.11.2019 06:31 SophieStar15
What is δe in kj for a system that receives 1.79 kj of heat from surroundings and has 4.51 kcal of work done on it at the same time. 1 cal = 4.184 j.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
What is δe in kj for a system that receives 1.79 kj of heat from surroundings and has 4.51 kcal of w...
Questions


Mathematics, 03.09.2020 14:01

English, 03.09.2020 14:01

Law, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01


Physics, 03.09.2020 14:01

Biology, 03.09.2020 14:01


Mathematics, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01



English, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01

English, 03.09.2020 14:01

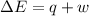
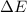 =Change in internal energy
=Change in internal energy
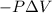 {Work is done on the system is positive as the final volume is lesser than initial volume}
{Work is done on the system is positive as the final volume is lesser than initial volume}
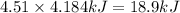 (1kcal = 4.184kJ)
(1kcal = 4.184kJ)