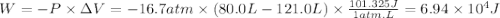Chemistry, 28.11.2019 05:31 loraine4664
Calculate the work associated with the compression of a gas from 121.0 l to 80.0 l at a constant pressure of 16.7 atm.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
Calculate the work associated with the compression of a gas from 121.0 l to 80.0 l at a constant pre...
Questions


Arts, 17.02.2022 14:00

English, 17.02.2022 14:00



Mathematics, 17.02.2022 14:00





History, 17.02.2022 14:00


Mathematics, 17.02.2022 14:00


Mathematics, 17.02.2022 14:00


History, 17.02.2022 14:00

Mathematics, 17.02.2022 14:00