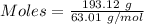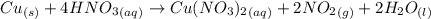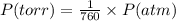Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
What volume of nitrogen dioxide is formed at 735 torr and 28.2 °c by reacting 3.56 cm3 of copper (d...
Questions


Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01




Geography, 29.09.2020 14:01


Mathematics, 29.09.2020 14:01


Biology, 29.09.2020 14:01

Health, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01

History, 29.09.2020 14:01

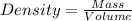
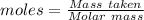
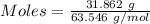
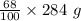 = 193.12 g
= 193.12 g