Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2


Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

You know the right answer?
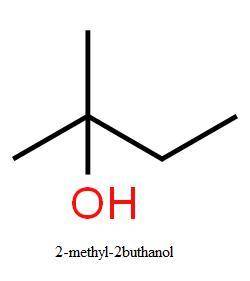
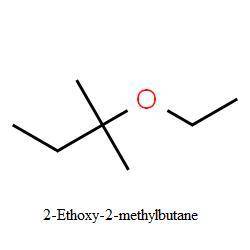
Gthe ratio of elimination to substitution is exactly the same (26% elimination) for 2−bromo−2−methyl...
Questions


Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


History, 18.03.2021 01:30








Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

English, 18.03.2021 01:30

Business, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30