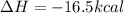Chemistry, 28.11.2019 03:31 cheating53
"in the absence of an adequate supply of oxygen, yeasts obtains metabolic energy by fermentation of glucose to produce ethanol. c6h12o6(s) latex: \longrightarrow⟶ 2 c2h5oh(l) + 2 co2(g) use the standard enthalpies of formation to calculate δh for this reaction" substance δho glucose(s) -304.5 kcal/mol co2(g) -93.9 kcal/mol c2h5oh(l) -66.4 kcal/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
"in the absence of an adequate supply of oxygen, yeasts obtains metabolic energy by fermentation of...
Questions



Mathematics, 01.09.2020 03:01


Physics, 01.09.2020 03:01

Mathematics, 01.09.2020 03:01

Spanish, 01.09.2020 03:01


Mathematics, 01.09.2020 03:01



History, 01.09.2020 03:01

Mathematics, 01.09.2020 03:01

Mathematics, 01.09.2020 03:01

English, 01.09.2020 03:01


Spanish, 01.09.2020 03:01

Mathematics, 01.09.2020 03:01


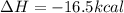
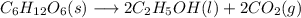
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0394/1940/76c37.png)
![\Delta H=[(n_{C_2H_5OH}\times \Delta H_{C_2H_5OH})+(n_{CO_2}\times \Delta H_{CO_2})]-[(n_{C_6H_{12}O_6}\times \Delta H_{C_6H_{12}O_6})]](/tpl/images/0394/1940/32cc8.png)
![\Delta H=[(2\times -66.4)+(2\times -93.9)]-[(1\times -304.5)]](/tpl/images/0394/1940/a8812.png)