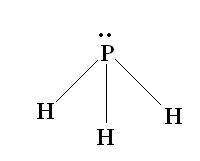
Determine the electron-group arrangement, molecular shape, and ideal bond angle for the following molecule: ph3 electron-group arrangement: tetrahedral trigonal pyramidal v-shaped trigonal planar molecular shape: tetrahedral trigonal pyramidal t-shaped bent ideal bond angle: degrees.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
Determine the electron-group arrangement, molecular shape, and ideal bond angle for the following mo...
Questions

Mathematics, 28.08.2020 22:01

English, 28.08.2020 22:01




Mathematics, 28.08.2020 22:01


Mathematics, 28.08.2020 22:01


Mathematics, 28.08.2020 22:01

Mathematics, 28.08.2020 22:01

Social Studies, 28.08.2020 22:01


English, 28.08.2020 22:01



Mathematics, 28.08.2020 22:01


Mathematics, 28.08.2020 22:01

Mathematics, 28.08.2020 22:01

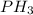 is trigonalbipyramidal and
is trigonalbipyramidal and 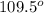 respectively.
respectively.