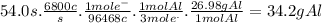Chemistry, 28.11.2019 00:31 angeljaylyn123
In the hall-heroult process, a large electric current is passed through a solution of aluminum oxide (a12o 3) dissolved in molten cryolite (na3aif6) ,resulting in the reduction of the ai�3 to pure aluminum. suppose a current of 6800.a is passed through a hall-heroult cell for 54.0 seconds. calculate the mass of pure aluminum produced. be sure your answer has a unit symbol and the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
In the hall-heroult process, a large electric current is passed through a solution of aluminum oxide...
Questions

Mathematics, 09.12.2020 16:10




English, 09.12.2020 16:10

Computers and Technology, 09.12.2020 16:10



Advanced Placement (AP), 09.12.2020 16:10

Mathematics, 09.12.2020 16:10



Physics, 09.12.2020 16:10


Mathematics, 09.12.2020 16:10

Mathematics, 09.12.2020 16:10

Biology, 09.12.2020 16:10

History, 09.12.2020 16:10