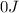Chemistry, 27.11.2019 22:31 harleypage308
Suppose 1.00 mol superheated ice melts to liquid water at 25°c. assume the specific heats of ice and liquid water have the same value and are independent of temperature. the enthalpy change for the melting of ice at 0°c is 6007 j mol21. calculate dh, dssys, and dg for this process.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
Suppose 1.00 mol superheated ice melts to liquid water at 25°c. assume the specific heats of ice and...
Questions




Chemistry, 03.08.2019 06:10


History, 03.08.2019 06:10













Spanish, 03.08.2019 06:20

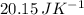
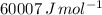
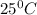
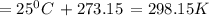
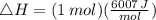
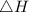 ) for the melting of 1.00 mole of ice at
) for the melting of 1.00 mole of ice at 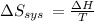
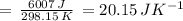
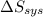 ) for the melting of 1.00 mole of ice at
) for the melting of 1.00 mole of ice at 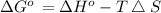
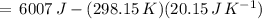
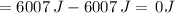
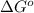 ) for the melting of 1.00 mole of ice at
) for the melting of 1.00 mole of ice at