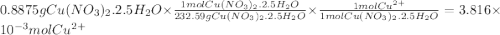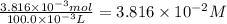Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 14:00
Which word refers to the smallest functional unit of living thing
Answers: 1

You know the right answer?
Astock solution of cu2+(aq) was prepared by placing 0.8875 g of solid cu(no3)2∙2.5 h2o in a 100.0-ml...
Questions



Biology, 04.08.2019 22:00

Advanced Placement (AP), 04.08.2019 22:00




Mathematics, 04.08.2019 22:00

Social Studies, 04.08.2019 22:00

Mathematics, 04.08.2019 22:00

Business, 04.08.2019 22:00

Chemistry, 04.08.2019 22:00

Social Studies, 04.08.2019 22:00

History, 04.08.2019 22:00

Biology, 04.08.2019 22:00

History, 04.08.2019 22:00



History, 04.08.2019 22:00